Phosphorus Mass Number
Phosphorus is a non-metal with diverse biological and industrial significance. It was discovered in 1669 by Hennig Brand and has two allotropic forms and eighteen isotopes.

Discovery and History
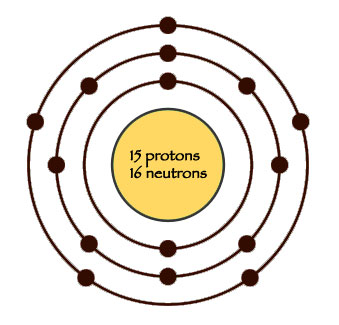
Phosphorus is a chemical element with atomic number 15 which means there are 15 protons and 15 electrons in the atomic structure. The chemical symbol for Phosphorus is P. Atomic Mass of Phosphorus Atomic mass of Phosphorus is 30.9738 u. Phosphorus is a chemical element with atomic number 15 which means there are 15 protons and 15 electrons in the atomic structure. The chemical symbol for Phosphorus is P. Atomic Mass of Phosphorus Atomic mass of Phosphorus is 30.9738 u. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
The discovery of phosphorus as a novel element was carried out by in Hennig Brand in 1669 by evaporating urine. He obtained a white material that burned brilliantly and emitted a glow in the dark when exposed to oxygen [1]. Antoine Lavoisier identified phosphorus as a separate element in 1777. The name phosphorus also has an interesting origin, as Phosphorus is the name of planet Venus in Ancient Greece language and it means “carrier of light” or “light-bringer”. Commercial scale production of phosphorus was started by Brand and later many scientists, including Robert Boyle, used the same method of phosphorus production developed by Brand. In 1680, Boyle introduced the use of phosphorus to ignite wooden splints with tips of sulfur, and these later were developed into matches [2]. Phosphorus was initially used on a wide scale to make poisons, explosives and nerve agents and due to its toxic and lethal properties, it was commonly named as the “devil’s element”. Gottlieb Gahn and Carl Wilhelm (1796) were the first to discover the presence of phosphorus, in the form of calcium phosphate, in the bones. Until 1840s, one of the major sources of phosphorus has been ashes of bones, and until 1840s. Later in 1850s, the presence of phosphate in rocks on the form of calcium phosphate was discovered and the phosphorus production switched form bones to rocks.
Phosphorus
Phosphorus atoms have 15 electrons and the shell structure is 2.8.5. The ground state electronic configuration of neutral phosphorus is Ne.3s 2.3p 3 and the term symbol of phosphorus is 4 S 3/2. Phosphorus: description Your user agent does not support the HTML5 Audio element. The other isotopes from mass 24 to mass 46 have been synthesized by appropriate nuclear reactions. All of these are radioactive with relatively short half-lives. The isotope of mass 32 has a half-life of 14.268 days and has proven extremely useful in tracer studies involving the absorption and movement of phosphorus in living organisms.
| Periodic Table Classification | Group 15 Period 3 |
|---|---|
| State at 20C | Solid |
| Color | Colourless, waxy white, yellow, scarlet, red, violet, black |
| Electron Configuration | [Ne] 3s2 3p3 |
| Electron Number | 15 |
| Proton Number | 15 |
| Electron Shell | 2, 8, 5 |
| Density | 1.82 g.cm-3 at 20°C |
| Atomic number | 15 |
| Atomic Mass | 30.97 g.mol -1 |
| Electronegativity according to Pauling | 2.19 |
Occurrence
Phosphorus does not exist in free or elemental state in nature. It forms various compounds and is present in large variety of minerals. The most common source of phosphorus is calcium phosphate that is present in rocks. Phosphorus is an element of great biological value. It is the 6th most abundant element in the living systems. The largest natural reserves of phosphorus are present in Arab region. Other countries or regions that are significant producers of phosphorus include Russia, China, Florida and Morocco [3]. The annual production of elemental phosphorus is around 1000,000 tons.
Physical Characteristics
White phosphorus is yellowish white solid, that has a waxy texture. Phosphorus undergoes spontaneous ignition in air and forms pentoxide (P4O10). There are two allotropic forms of phosphorus, red and black, that differ in physical and chemical properties. Red phosphorus (which is formed by heating of white phosphorus at high temperature) ignites on friction. White phosphorus is a highly toxic substance., while red phosphorus is non-toxic [4]. Phosphorus is water insoluble. Black phosphorus is also termed as violet phosphorus and is the least reactive allotrope of phosphorus. It resembles graphite in appearance and structure.
Chemical Characteristics
White phosphorus is very reactive element. In the presence of oxygen, white phosphorus emits a light green glow, termed as chemiluminescence (glow caused by cold chemical reaction) [5]. The most abundant compounds of phosphorus contain the tetrahedral anion of phosphate (PO43-). There is a vast variety of phosphorus compounds including oxoacids (phosphoric acid), sulfides, nitrides (phosphorus nitride halogens (F2PN, Cl2PN), and phosphides (reaction of metals with red phosphorus). Phosphine (PH3) is a toxic compound with pungent smell, is structural analogue of ammonia. Diphosphine (P2H4), is an analogue of hydrazine and is highly flammable.

Uses and Significance
- The largest use of phosphorus is in the production of fertilizers. It is an essential nutrient for the growth of plants.
- Phosphorus is used in making of safety matches, and various ammunitions, such as incendiary shells and hand grenades etc.
- Phosphorus is used in the manufacturing of bronze and steel.
- It is used in making LEDs (light-emitting diodes).
- Phosphorus is used in making detergents, that functions to remove water hardness and improve the efficiency of detergent.
- It is used in synthesis of nerve agents.
- It is used as active ingredient in various pesticides.
- Phosphoric acid is widely used in manufacturing of soft drinks, baking powder.
- Various compounds of phosphate are used in processing of cheese and meat.
Health Hazards
Phosphorus in the form of phosphate is a vital compound for all living systems. The energy currency of cell, ATP (adenosine triphosphate) that regulates every process in the living cell, uses phosphate. Phosphorylation, that is the process of adding phosphate to various biological molecules, is an important regulatory mechanism in living organisms. Lipids in combination with phosphorus (phospholipids) are the primary building blocks of cell membrane. Phosphorus is present in the building blocks of RNA and DNA. A balanced diet includes a definitive everyday intake of phosphorus. About 0.7 kg of phosphorus is present in an average adult human being, mostly in teeth and bones and in soft tissues of the body. deficiency of phosphate in the body can lead to various physiological effects, including tissue weakness, neurological defects and lack of ATP. Increased intake of phosphate can cause hardening of tissues and organs and diarrhea.
:max_bytes(150000):strip_icc()/human-body-composition-59021b3c5f9b5810dc784933.jpg)
Isotopes of Phosphorus
There are twenty-three isotopes of phosphorus, that range in atomic numbers from 26 to 43. Natural phosphorus constitutes only one stable isotope, phosphorus-31 [6].
REFERENCES
[1]. Beatty, Richard (2000). Phosphorus. Marshall Cavendish. p. 7. ISBN0-7614-0946-7.
[2]. Peter Baccini; Paul H. Brunner. Metabolism of the Anthroposphere. MIT Press, 2012. p. 288. ISBN0262300540.
[3]. Philpott, Tom (March–April 2013). “You Need Phosphorus to Live—and We’re Running Out”. Mother Jones.fwhi
[4]. Abundance. ptable.com
[5]. Michael A. Sommers. Phosphorus. The Rosen Publishing Group, 2007. p. 25. ISBN1404219609.
Other Periodic Table Elements
- Tennessine
Tennessine is a synthetic element that was discovered in 2010. It is highly radioactive and…
- Moscovium
Moscovium is a synthetic element that was discovered in 2003. It is a highly radioactive…
- Hassium
Hassium is a synthetic element that was discovered in 1978. It is a highly radioactive…
The Element Phosphorus
[Click for Isotope Data]
Atomic Number: 15
Atomic Weight: 30.973761998
Melting Point: 317.30 K (44.15°C or 111.47°F)
Phosphorus Atomic Weight Test
Boiling Point: 553.65 K (280.5°C or 536.9°F)
Density: 1.82 grams per cubic centimeter
Phase at Room Temperature: Solid
Element Classification: Non-metal
Period Number: 3
Group Number: 15
How Many Electrons Are In Phosphorus
Group Name: Pnictogen
What's in a name? From the Greek word for light bearing, phosphoros.
Say what? Phosphorus is pronounced as FOS-fer-es.
History and Uses:
In what is perhaps the most disgusting method of discovering an element, phosphorus was first isolated in 1669 by Hennig Brand, a German physician and alchemist, by boiling, filtering and otherwise processing as many as 60 buckets of urine. Thankfully, phosphorus is now primarily obtained from phosphate rock (Ca3(PO4)2).
Phosphorus has three main allotropes: white, red and black. White phosphorus is poisonous and can spontaneously ignite when it comes in contact with air. For this reason, white phosphorus must be stored under water and is usually used to produce phosphorus compounds. Red phosphorus is formed by heating white phosphorus to 250°C (482°F) or by exposing white phosphorus to sunlight. Red phosphorus is not poisonous and is not as dangerous as white phosphorus, although frictional heating is enough to change it back to white phosphorus. Red phosphorus is used in safety matches, fireworks, smoke bombs and pesticides. Black phosphorus is also formed by heating white phosphorus, but a mercurycatalyst and a seed crystal of black phosphorus are required. Black phosphorus is the least reactive form of phosphorus and has no significant commercial uses.
Phosphoric acid (H3PO4) is used in soft drinks and to create many phosphate compounds, such as triple superphosphate fertilizer (Ca(H2PO4)2·H2O). Trisodium phosphate (Na3PO4) is used as a cleaning agent and as a water softener. Calcium phosphate (Ca3(PO4)2) is used to make china and in the production of baking powder. Some phosphorus compounds glow in the dark or emit light in response to absorbing radiation and are used in fluorescent light bulbs and television sets.
Estimated Crustal Abundance: 1.05×103 milligrams per kilogram
Estimated Oceanic Abundance: 6×10-2 milligrams per liter
Number of Stable Isotopes: 1 (View all isotope data)
Phosphorus Protons Neutrons Electrons
Ionization Energy: 10.487 eV
Oxidation States: +5, +3, -3
Mass Number Of Phosphorus 30
Electron Shell Configuration: | 1s2 |
2s2 2p6 | |
3s2 3p3 |
Phosphorus Neutrons Mass Number
For questions about this page, please contact Steve Gagnon.
